
Enteric resin (L 100)
pH-dependent controlled release excipients, insoluble in water, and low permeability.
It can be used for sustained-release enteric coating.
Swell at pH <6.0 and dissolve at pH >6.0 (Jejunum targeting).
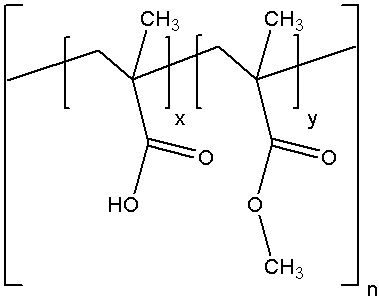
Chemical name:
Methyl methacrylate-methyl methacrylate copolymer (weight ratio ≈1:1)
中文名:
甲基丙烯酸共聚物A型
聚丙烯酸树脂II (CP2015)
English name:
Methacrylic Acid and Methyl Methacrylate Copolymer (1:1) (NF36)
Methacrylic Acid - Methyl Methacrylate Copolymer (1:1) (EP9.0)
Japanese name:
メタクリル酸コポリマーL(医薬品添加物規格)
CDE Registration No.:F20200000241
CAS: 25086-15-1
UNII: 74G4R6TH13
Packing specification: 10kg/barrel
Quality specification:CP、USP、EP、JPE
Physical properties
Properties: white granules or powder (L 100); slightly odor.
Mw: ≈125,000 g/mol
Tg: not available (pyrolysis)
Extensibility: very poor (plasticizer with 40-50% copolymer weight).
Chemical properties
Anionic property, weakly acidic;
pH <6.0 swell, permeability; pH > 6.0 dissolution (jejunal release).






